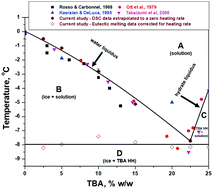
A refined phase diagram of the tert-butanol–water system and implications on lyophilization process optimization of pharmaceuticals - Physical Chemistry Chemical Physics (RSC Publishing)

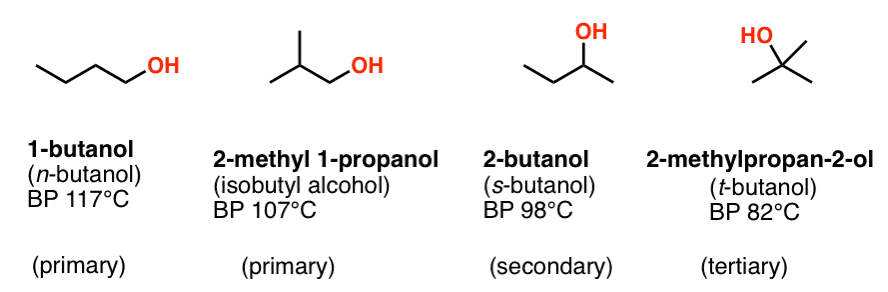
Arrange the following compounds in order of their increasing boiling points.n - butyl alcohol, glycerol, n - butane, tert - butyl alcohol, sorbitol, n - butyraldehyde, isobutyl alcohol.

Impact of a tert-butyl alcohol-cyclohexane system used in unidirectional freeze-casting of SiOC on compressive strength and mass transport - ScienceDirect
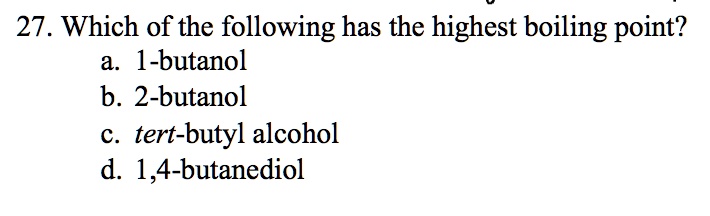
SOLVED: 27. Which of the following has the highest boiling point? 1-butanol b. 2-butanol C tert-butyl alcohol d. 1,4-butanediol