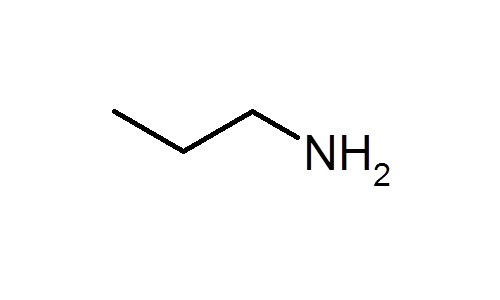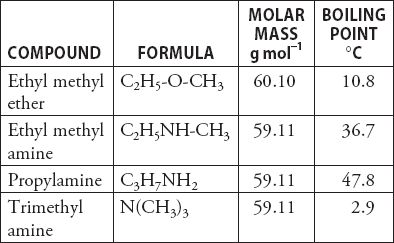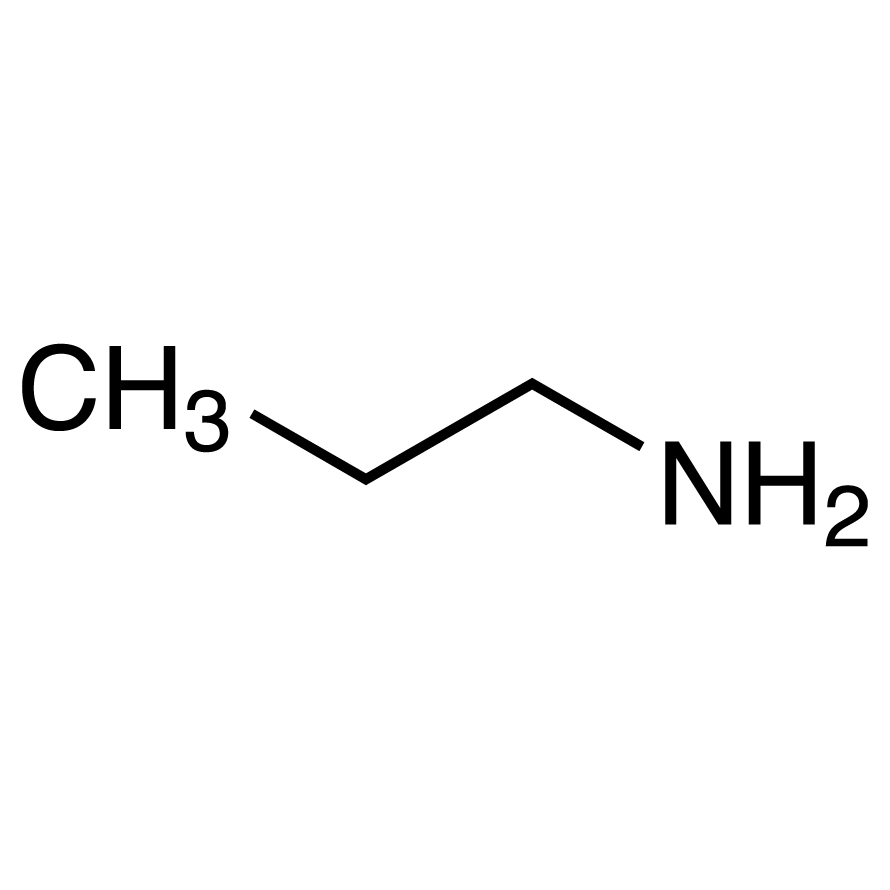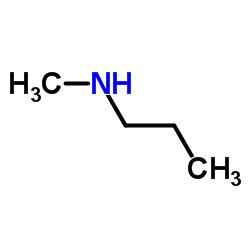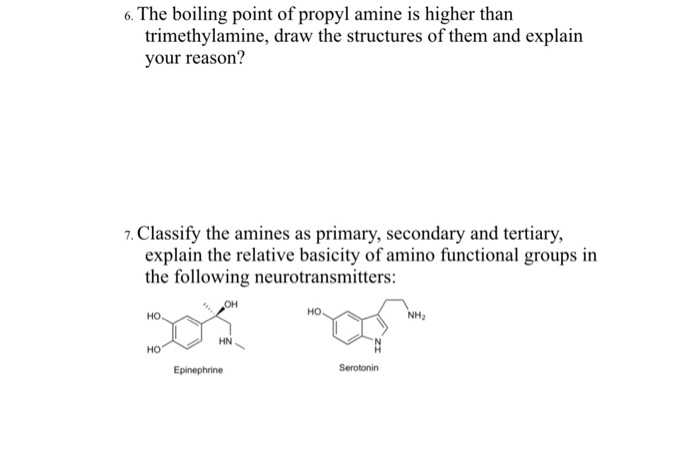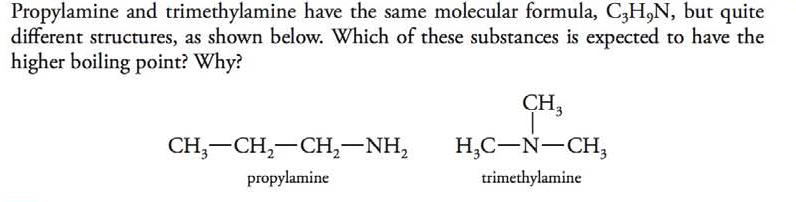
Propylamine and trimethylamine have the same molecular formula, C 3 H 9 N, but quite different structures, as shown below. Which of these substances is expected to have the higher boiling point?

Among the following amines namely ethylmethyl amine, propyl amine trimetyl amine, the lowest boi... - YouTube

Linear aliphatic primary amines melting points boiling points solubility in water hydrogen bonding structure classification physical properties of aliphatic amines organic nitrogen compounds advanced A level organic chemistry revision notes doc brown
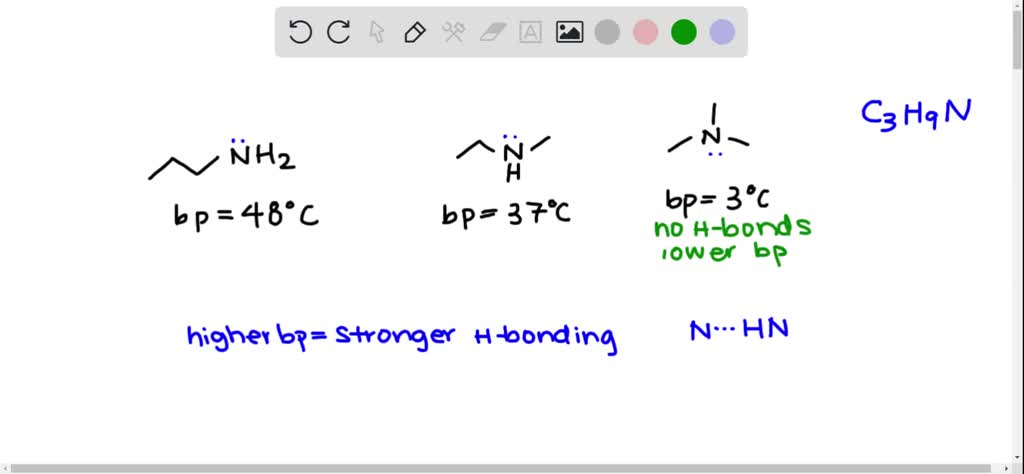
SOLVED:Propylamine (bp 48^∘ C ), ethylmethylamine (bp 37^∘ C ), and trimethylamine (bp 3^∘ C ) are constitutional isomers with the molecular formula C3 H9 N. Account for the fact that trimethylamine


-propylamine[818550_(Trimethoxysilyl)-propylamine-ALL].jpg)