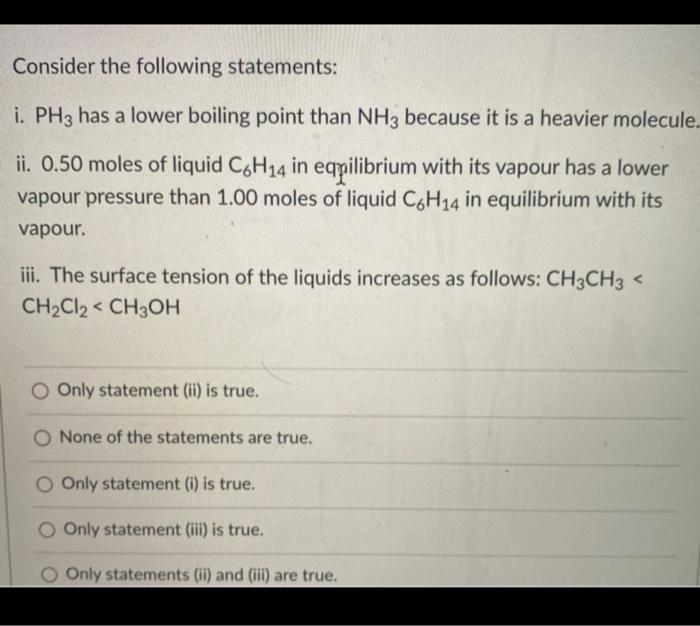
No links please Q NH3 has higher boiling point than PH3, explain - Chemistry - Solutions - 12503991 | Meritnation.com

Among the following, which has the highest boiling point? (A) NH3 (B) PH3 (C) AsH3 (D) CH - Brainly.in

SOLVED: Which is the correct order regarding the boiling points of hydrides of Group 15? Select one: a. NH3 PHa AsH3 b. PH3 AsH3 NH3 c. NH3 AsH3 PHa d. PH3 NH3 <
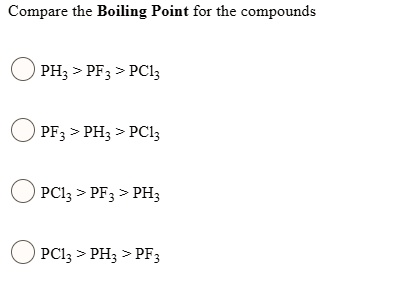
SOLVED: Compare the Boiling Point for the compounds PH; > PF3 > PCl; PF3 > PH; > PCl; PCl; > PF3 PH; PCl; > PH; > PF3

The hydrides of group 5A are NH3, PH3, AsH3, and SbH3. Arrange them from highest to lowest boiling point. - Brainly.com

Solve this: li) Why PH3 has lower boiling point than NH3 - Chemistry - The p-Block Elements - 11742649 | Meritnation.com

What is the Order of melting point in nh3 sbh3 ash3 ph3 and why plz answer fast - Chemistry - Chemical Bonding and Molecular Structure - 12901659 | Meritnation.com
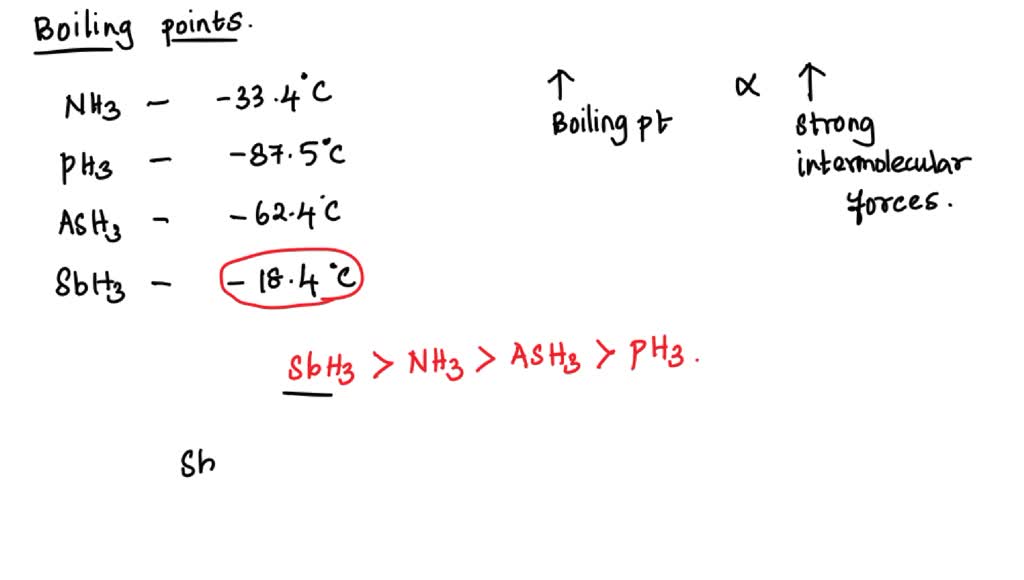







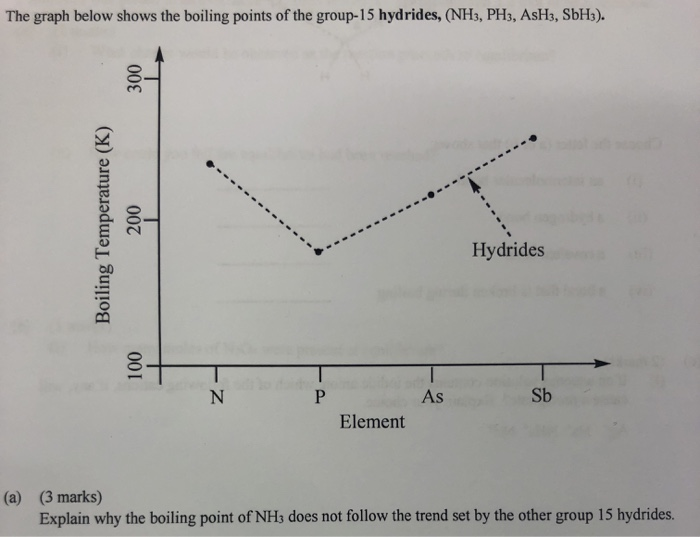
![Solved] NH3 has a much higher boiling point than PH3 because Solved] NH3 has a much higher boiling point than PH3 because](https://storage.googleapis.com/tb-img/production/21/01/F1_Utkarsha.S_30-01-21_Savita_D13.png)