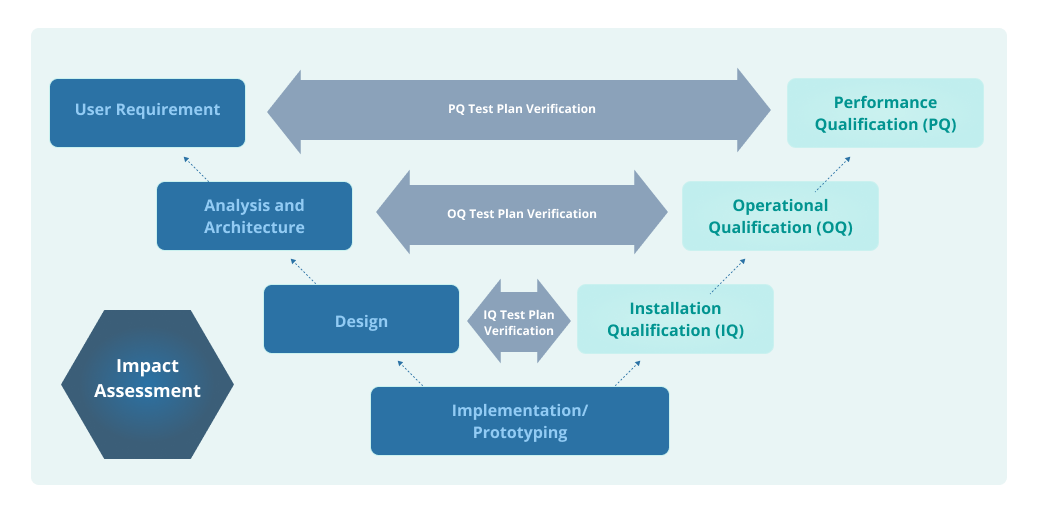
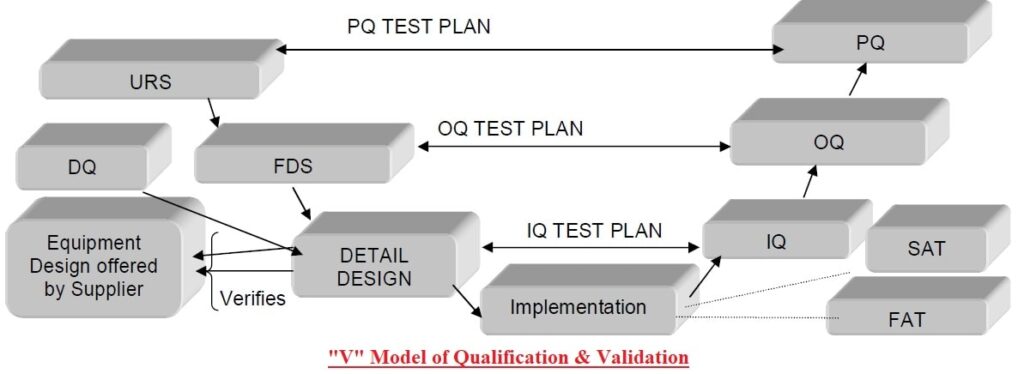
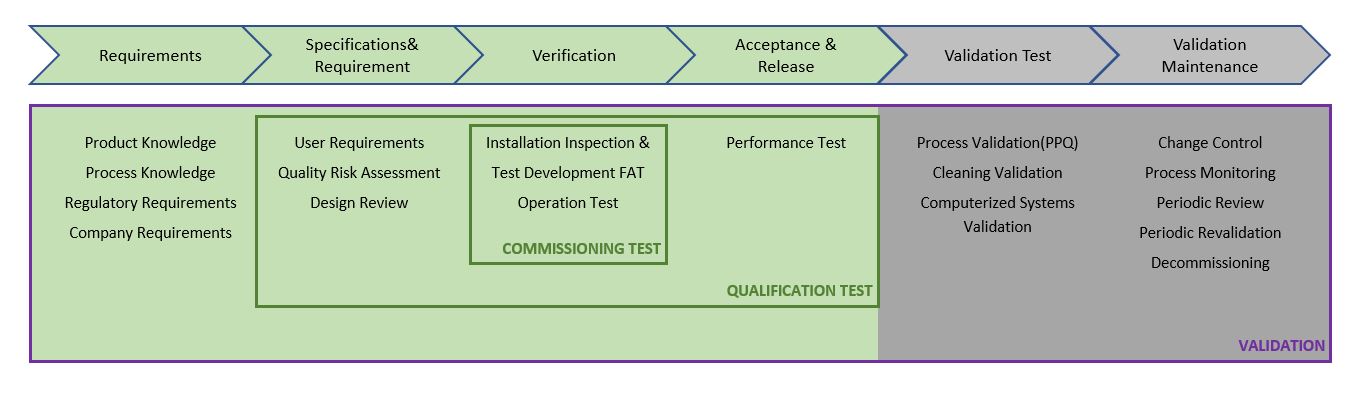
PPT – Instrument Qualification DQ, IQ, OQ, PQ vs. Validation and Routine MaintenanceCalibration PowerPoint presentation | free to view - id: 4081a-MTU0N

IQ OQ PQ | Process Validation | Equipment Validation | Equipment Qualification | Medical Devices - YouTube