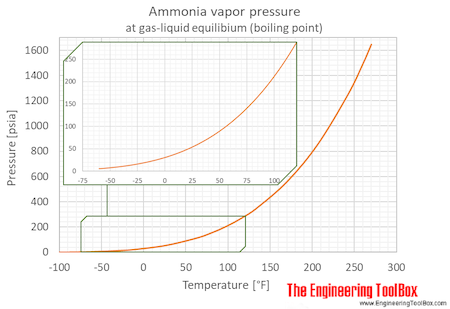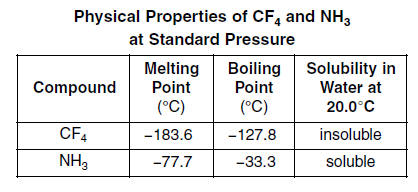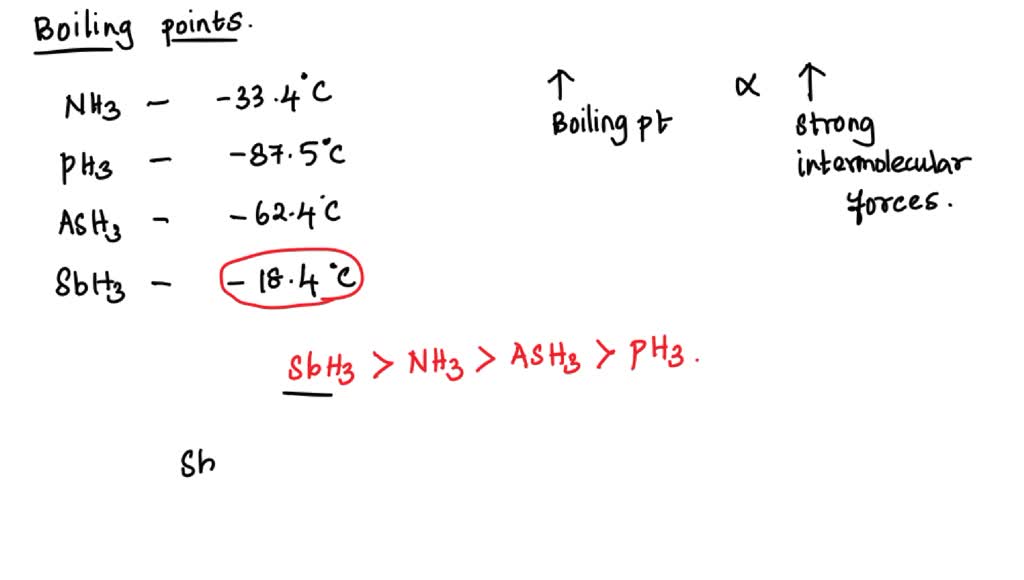
SOLVED: The boiling point of NH3, PH3,AsH3 and SbH3 are respectively -33.4 oC,-87.5 oC, -62.4 oC, -18.4oC. Explain the variation of their boiling points in terms of the types of intermolecular forces.

A diagram illustrating the "unexpected" rise in boiling point (Y-axis)... | Download Scientific Diagram


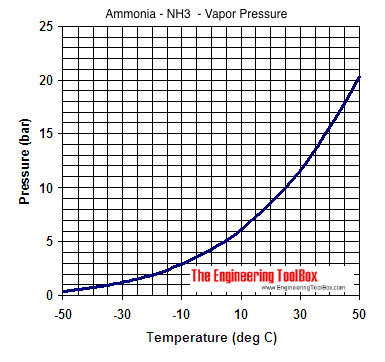





![Solved] NH3 has a much higher boiling point than PH3 because Solved] NH3 has a much higher boiling point than PH3 because](https://storage.googleapis.com/tb-img/production/21/01/F1_Utkarsha.S_30-01-21_Savita_D13.png)