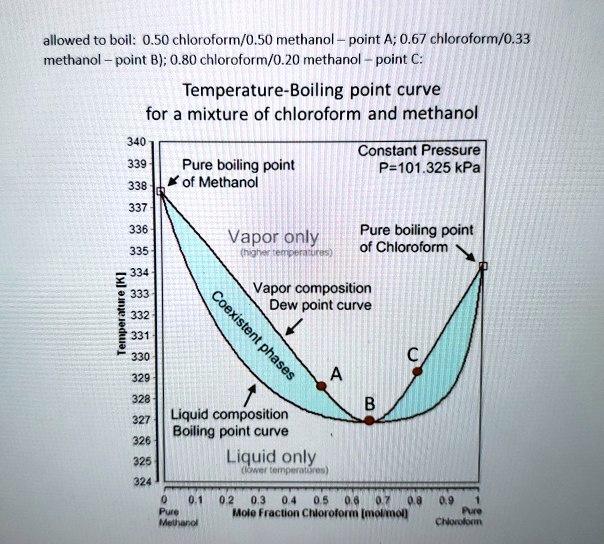
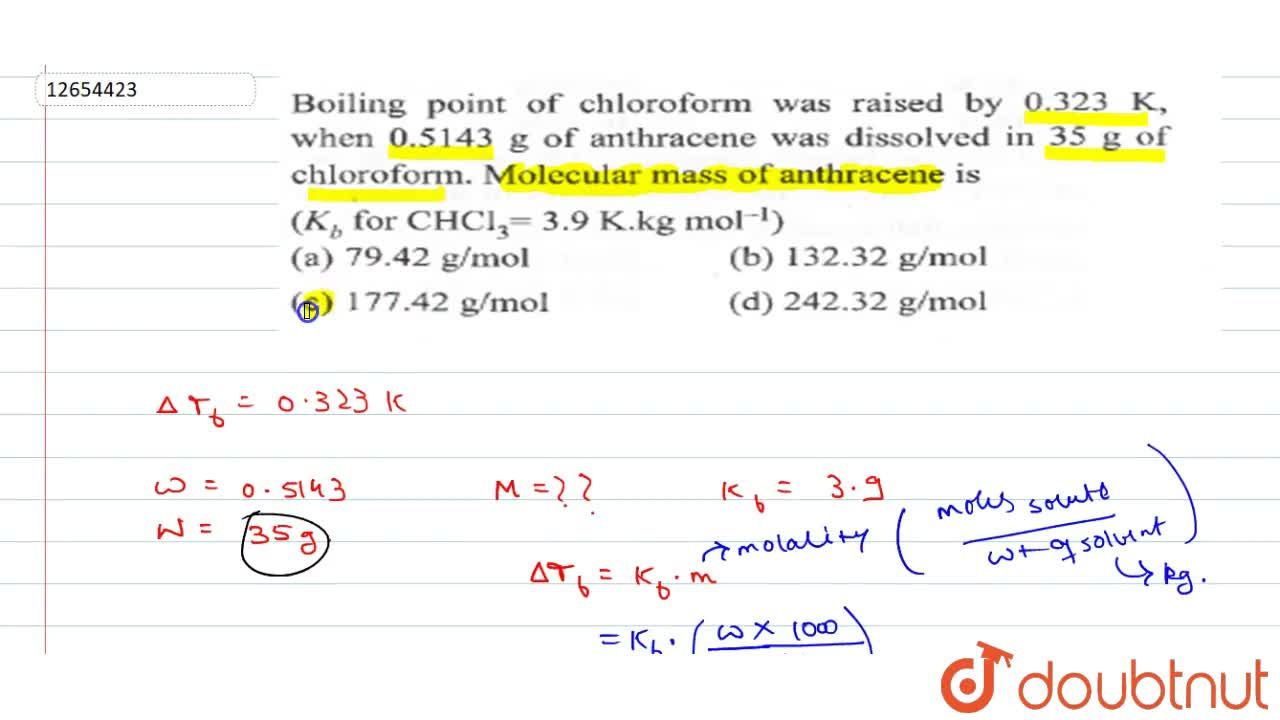
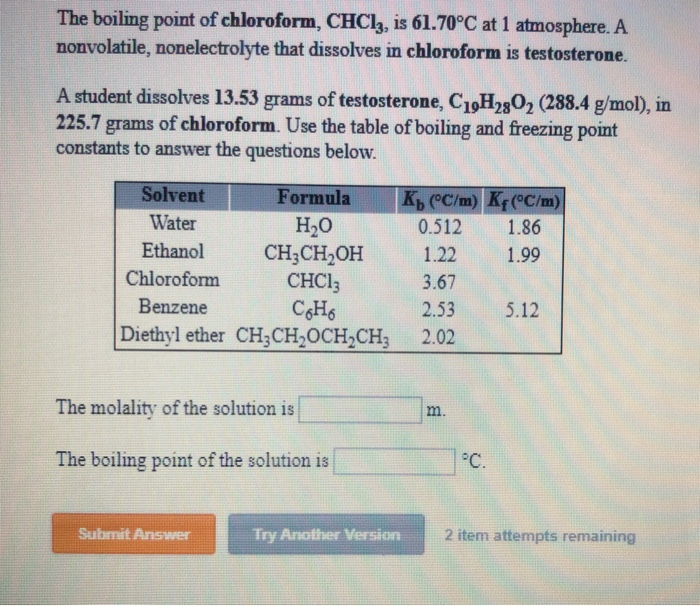
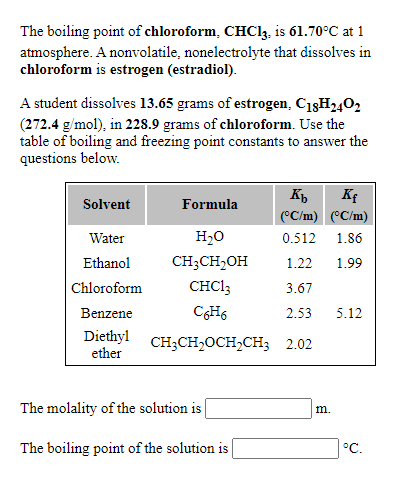
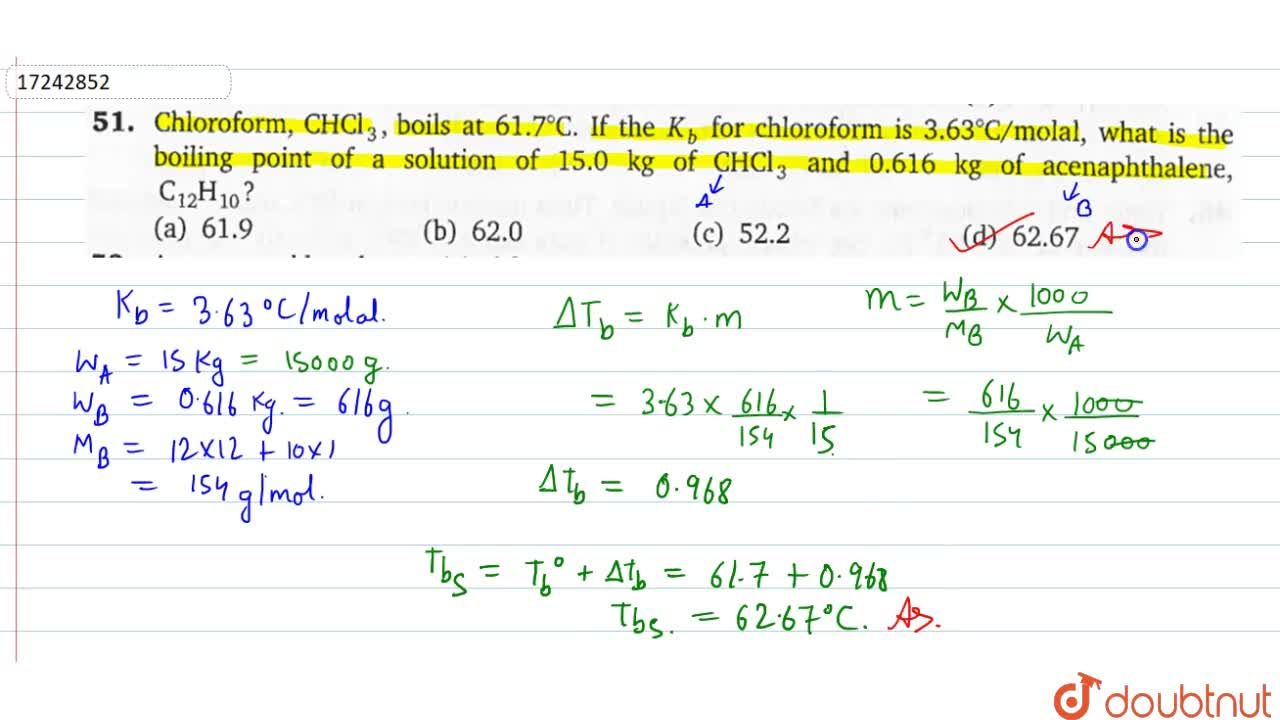
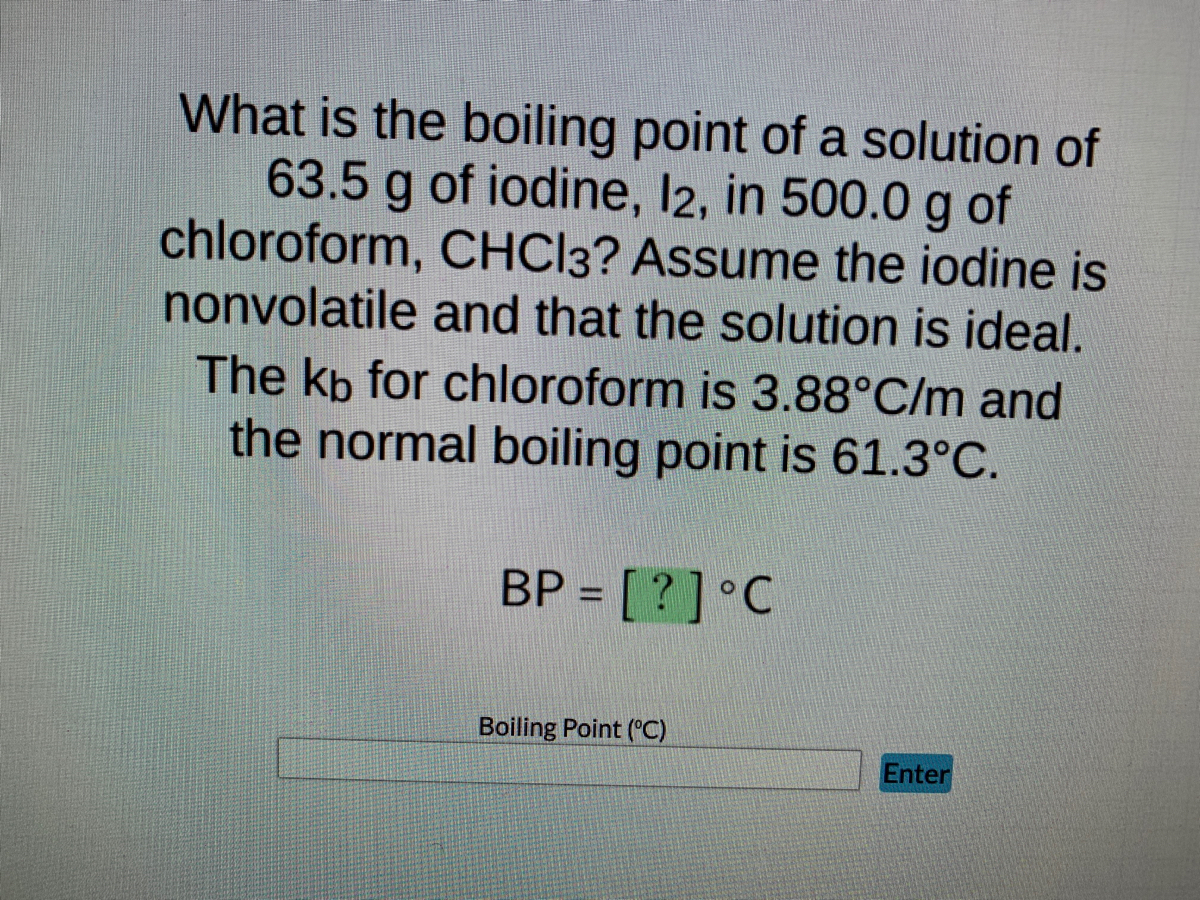
45. Boiling point of chloroform was raised by \( 0.323 K \), when \( 0.5143 g \) of anthracene was dissolved in \( 35 g \) of chloroform. Molecular mass of anthracene

OneClass: Using data from the table, calculate the freezing and boilingpoints of each of the follow...
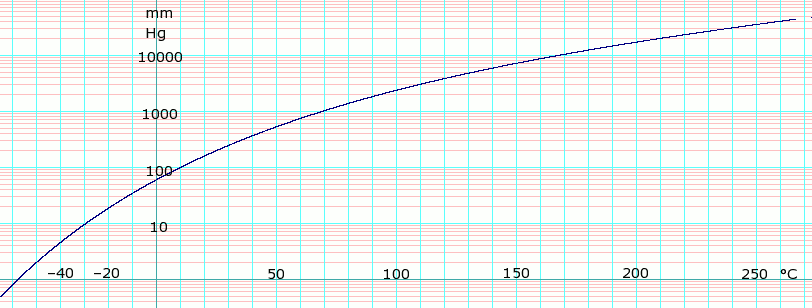
What is the normal boiling point of chloroform if its heat of vaporization is 31.4 kJ/mol and it has a vapor pressure of 190.0 mmHg at 25.0 °C? - Quora

Calculate molal boiling point constant for chloroform if it's boiling point is 61 2 degree celsius , molality = 0 - Chemistry - Solutions - 12555779 | Meritnation.com

Why is the boiling point of trichlorofluoromethane lower than that of chloroform? - Chemistry Stack Exchange
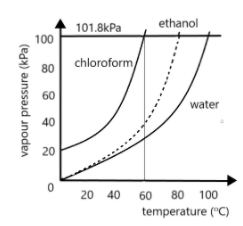
What is the normal boiling point for chloroform?\n \n \n \n \n A. $40^\\circ C$B. $50^\\circ C$C. $60^\\circ C$D. $70^\\circ C$E. $80^\\circ C$
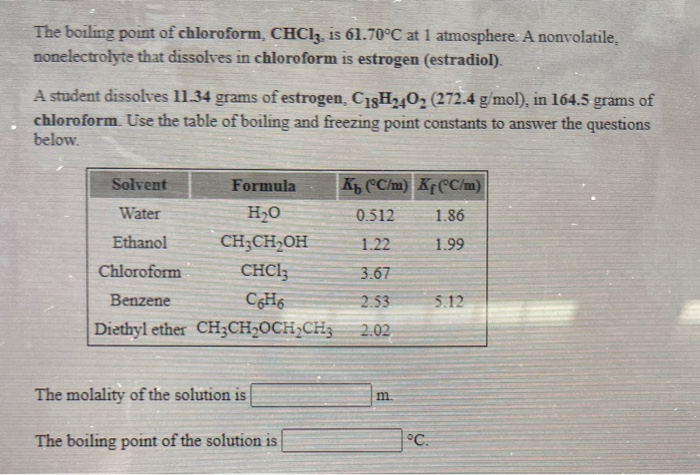
57. calculate bp of solution cntaining 25g urea 25g thio urea in 500g of chloroform boiling point of pure chloroform is 61.2 degree celsius Kb = 3.63